IRES constructs redux
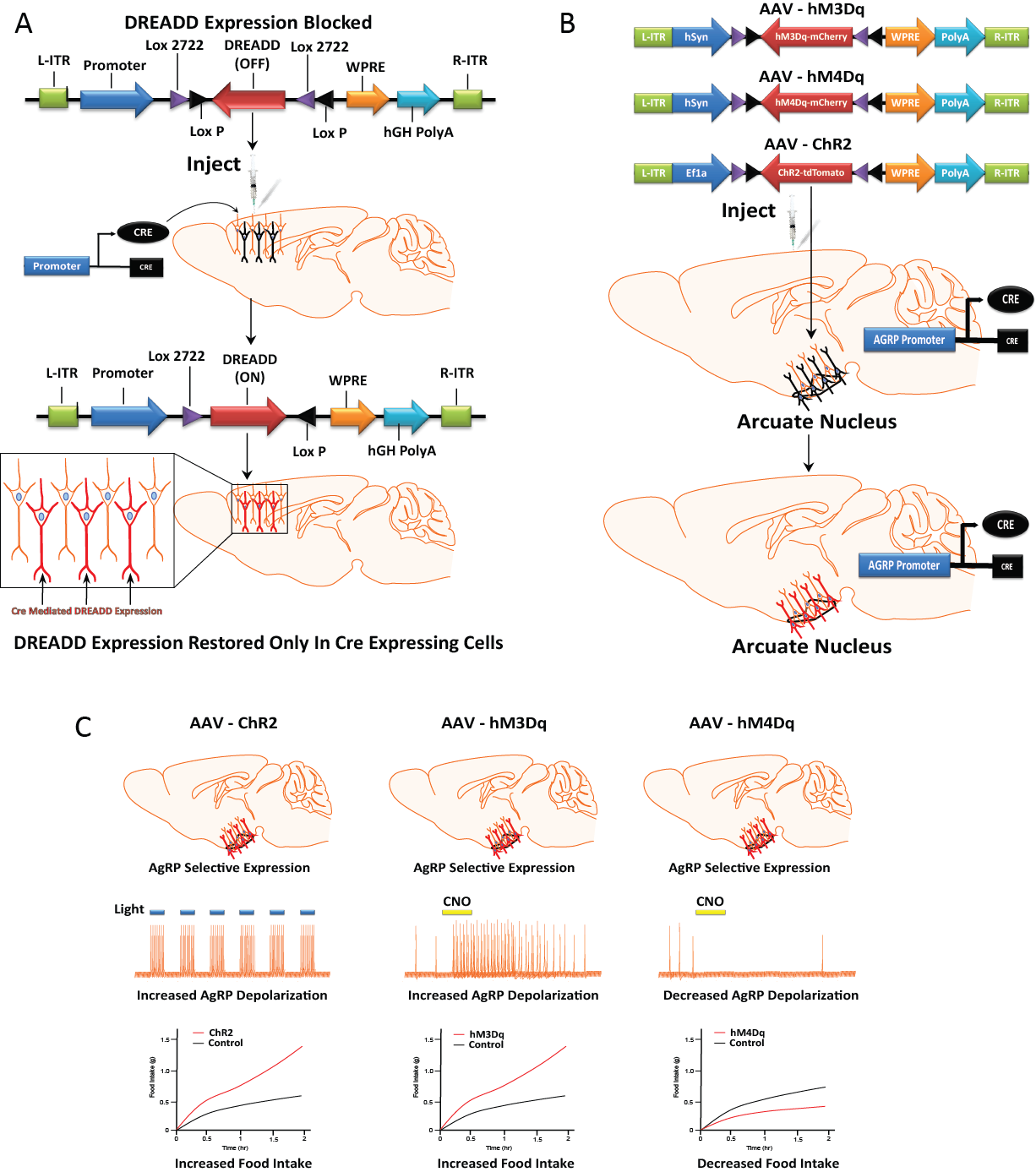
I've received quite a few emails regarding my decision to no longer offer high titer viral stocks of the various IRES constructs via the UNC vector core. And thought I'd clarify for those interested.... The constructs work beautifully in our hands (see for instance our recent paper where we used the CAMKII-hM4Di-IRES-mCitrene vector ) and yield highly reproducible and high levels of transduction in mice. I have received a small number of emails from users who have not been able to reproducibly achieve high levels of transduction when the DIO-hSyn-DREADD-IRES-mCitrene constructs are used in combination with a Cre-driver line (even though they function well in our hands). My sense is that there was some variability on the packaging on the part of the vector core and I directed them to take them off line. The plasmids are/will be available from ADDGENE. Bryan